Exploring TIGIT expression in pediatric dialysis patients with a stable-like phenotype and implications for belatacept-resistant rejection
Charlotte Duneton1,2, Roshan George2, Rouba Garro2, Julien Hogan1,4, Mandy Ford5, Guislaine Carcelain6,7.
1Pediatric Nephrology, dialysis and transplantation department, Robert Debré University Hospital, APHP, Paris, France; 2INSERM U976, Université Paris Cité, Paris, France; 3Pediatric Nephrology Department, Emory School of Medicine , Children’s Healthcare of Atlanta, Atlanta, United States; 4Paris Translational Research Center for Organ Transplantation, INSERM, UMR-S970 , Université Paris Cité, Paris, France; 5Emory Transplant Center and Department of Surgery , Emory University, Atlanta, United States; 6Immunology Laboratory, Robert Debré University Hospital, APHP, Paris, France; 7INSERM UMR1141, Université Paris Cité, Paris, France
Background: Belatacept (CTLA4-Ig) improves graft and patient survival in KT. However, a subset of patients receiving belatacept presents an increased risk of cellular rejection compared to CNI patients. Studies from Emory University showed that, in adults, a high proportion of CD28+CD4+ effector memory T cells (TEM) before KT is associated with a higher risk of rejection. Conversely, stable patients on belatacept exhibit a lower proportion of these cells and decreased cytokine production, suggesting a link to immune senescence. Our preliminary data show that these phenotypes also exist in pediatric patients.
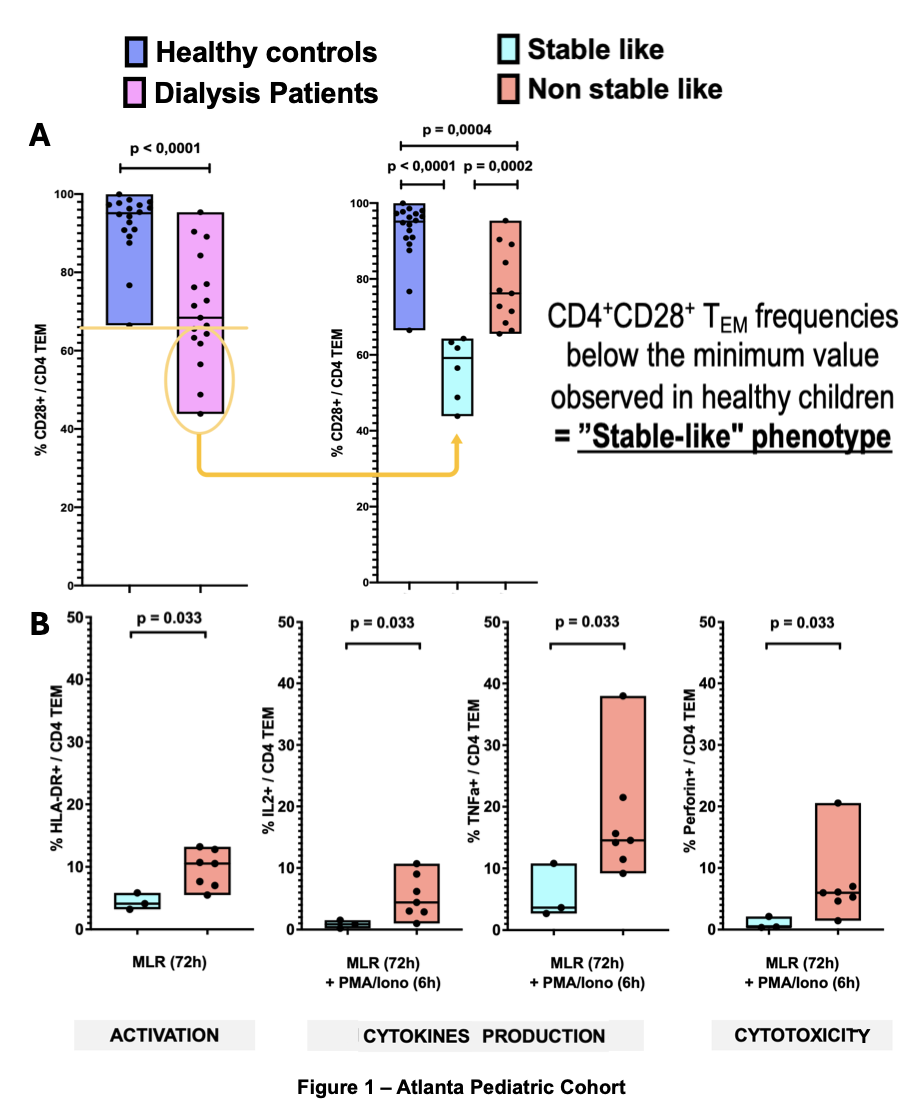
Despite their young age, a subset of children on dialysis accumulates CD4+TEM cells that have lost CD28 expression, a phenotype similar to stable adults on belatacept. Stable-like patients show decreased functional capacities in response to allogenic stimulation (Fig 1B).
Objective: TIGIT is a well-characterized inhibitory receptor in immuno-oncology, yet its role in transplantation remains largely unknown. We aimed to explore the role of TIGIT in patients with the stable-like phenotype.
Methods: We examined TIGIT expression in dialysis patients at Robert Debré Hospital, Paris, and healthy controls. Peripheral blood mononuclear cells (PBMCs) were isolated and analyzed using multiparameter flow cytometry to assess TIGIT expression. Additional markers of T cell activation and functional capacity were also evaluated.
Results: The median age of dialysis patients (n=19) was 10.6 years, compared to 8.6 years in healthy controls (n=4, p=0.68). Among the dialysis cohort, 8 patients exhibited a stable-like phenotype, characterized by an accumulation of CD4+ TEM cells lacking CD28 expression, while 11 did not (non-stable-like group). There were no significant differences between these groups regarding age, underlying renal disease etiology, or time on dialysis. Notably, none of the dialysis patients had been previously exposed to immunosuppressive therapy. TIGIT expression was significantly higher in stable-like patients within the CD4+ T cell population compared to both non-stable-like patients and healthy controls.
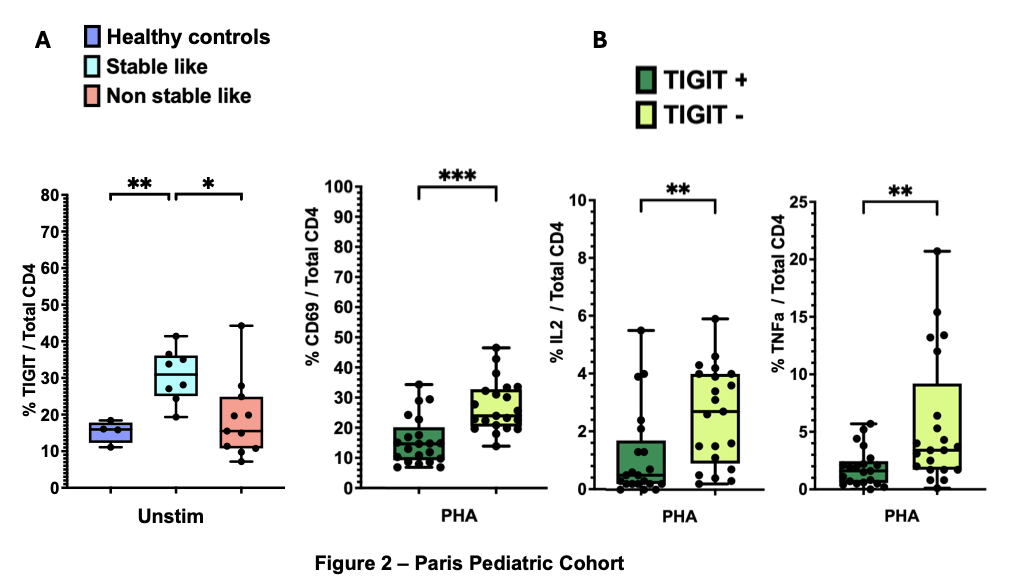
Functionally, CD4+TIGIT+ T cells exhibited diminished responses to stimulation, as evidenced by reduced expression of the activation marker CD69 and lower intracellular production of IL-2 and TNF-α following phytohemagglutinin (PHA) stimulation (Fig 2B).
Conclusion: Interestingly, TIGIT expression was not only elevated in stable-like patients compared to non-stable-like patients but also higher than in healthy controls. This finding leads us to hypothesize that premature immune senescence may contribute to this phenotype. Further investigations are needed to assess the co-expression of PD-1, CD57, TIM3, and LAG3 on these cells to better characterize their differentiation status. These findings also suggest that TIGIT agonism could represent a novel therapeutic approach to mitigate belatacept-resistant rejection.
[1] Kidney transplantation
[2] Belatacept
[3] Memory T cell
[4] TIGIT