Intra-operative flow rates in technical variant pediatric liver transplantation: Measurement distribution and a potential predictor of post-operative vascular complications – A Starzl Network Initiative
Renana Yemini1,2, George Mazariegos1,2, Kyle Soltys1,2, Jonathan Merola1,3.
1Starzl Network for Excellence in Pediatric Transplantation, UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, United States; 2Hillman Center for Pediatric Transplantation, Thomas E. Starzl Transplantation Institute, UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, United States; 3Division of Pediatric General and Thoracic Surgery, Cincinnati Children's Hospital Medical Center, University of Cincinnati, Cincinnati, OH, United States
Background: Transit time flow measurement to measure portal venous and hepatic arterial flow during pediatric liver transplantation has not been well studied but may inform the need for modulatory maneuvers to minimize vascular complications after liver transplantation. We utilized data collected from the Starzl Network, a collaborative learning network capturing >70% of pediatric transplant volume in North America, to evaluate the distribution of portal and arterial flows following liver transplantation in a pilot project assessing pediatric patients and their association to thrombotic post-transplant events.
Methods: Data entered on patients <18-year-old undergoing liver transplantation in the Starzl Network who had intra-operative portal venous and arterial flow and graft weight measurements recorded from 2020-2024 were included in this analysis. Outcomes of portal vein and hepatic arterial thrombosis as well as graft loss were assessed.
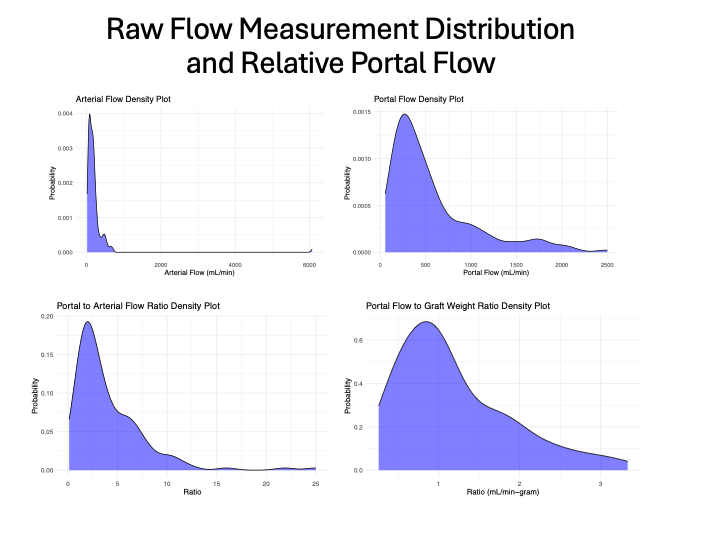
Results: 66 pediatric liver transplant recipients from 9 centers were included among which 29 (44%) were living donors and 55 (86%) were technical variant grafts. Flow distribution is shown in Figure 1. Four hepatic artery (6%) and two portal vein thromboses (3%) occurred in this cohort over a median follow-up of 1 year. There were three graft losses over this period (5%), though none were resultant from vascular complications. The average portal flow/graft weight and hepatic artery/graft weight ratios were 1.2 cc/min/g and 0.37cc/min/g, respectively. The average portal/arterial flow ratio was 4.6. 3 of the 4 (75%) arterial thromboses notably occurred when the hepatic artery/graft weight ratio was <0.25 and 2 (50%) occurred in patients in which the portal vein/hepatic artery flow ratio was >10.
Conclusions: Collective data from pediatric learning networks can help establish expected portal venous and arterial flow distributions in pediatric liver transplant. Ranges outside the norm may portend higher rates of vascular complications, which may be salvaged with portal modulatory maneuvers, though low event rates limit interpretation at present. Future studies will collect additional data on flow rates prospectively to assess the impact on vascular outcomes.