Inhibition of HDAC-6 mitigates acetaminophen-induced hepatotoxicity
Lanette Christensen1, Ciaran O'Brien1, Paul Hernandez1, Seth Concors3, Guanghui Ge1, Zhonglin Wang1, Wayne W Hancock1,2, Matthew H Levine1,2.
1Department of Surgery, University of Pennsylvania, Philadelphia, PA, United States; 2Pathology and Laboratory Medicine, Children's Hospital of Philadelphia, Philadelphia, PA, United States; 3Department of Surgery, Emory University, Atlanta, GA, United States
Introduction: Acetaminophen (APAP)-induced hepatotoxicity is among the leading causes of global pediatric acute liver injury (ALI), which can lead to acute liver failure (ALF). N-acetylcysteine (NAC) therapy is standard of care treatment for APAP-mediated ALI and reduces mortality, however has a narrow effective window after injury after which supportive care and subsequent liver transplantation for refractory injury remains the only therapy. Inhibition of the histone deacetylase, HDAC-6 has demonstrated protection in other liver injury models like ischemia-reperfusion injury and is an attractive therapeutic target having limited off-target effects. Here, HDAC-6 inhibition was evaluated as a therapy for acetaminophen-induced liver injury (AILI).
Methods: AILI was assessed in C57BL/6 (WT) mice that were given a sublethal dose of APAP (500mg/kg) following overnight fasting. WT mice were administered NAC (150 mg/kg), the HDAC-6 inhibitor Tubastatin A (TubA) (40 mg/kg), a combination of NAC and TubA, or vehicle control either as pre-treatment at 16 hrs and 1 hr prior to APAP, or as therapy 1, 3, or 5 hrs post-APAP injury. Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and glutathione were measured 24 hrs post-injury. Histopathological analysis by Modified Suzuki scoring and mRNA expression by RT-qPCR were used to evaluate livers 24 hrs post-injury.
Results: Pre-treatment with HDAC-6 inhibitor, TubA, provided significant protection against APAP toxicity, both independently and in combination with NAC. TubA in combination with NAC did not improve the protection provided by TubA alone. This was demonstrated by reduced AST (Fig. 1a) and ALT (Fig. 1b) serum levels and reduced histopathologic liver damaged (p<0.05). TubA pre-treatment provided prolonged survival post-APAP toxicity (p<0.0001). TubA pre-treatment resulted in reduced mRNA expression of inflammatory factors IFN-g and IL-6, and oxidative response mediators NOS2, Nrf2, and Hmox1 as compared to vehicle-treated controls (p<0.05). Importantly, reduced (effective) hepatic glutathione levels remained unchanged after APAP toxicity with TubA pre-treatment as compared to fasting controls, while levels of those treated with NAC or vehicle were depleted (Fig. 1c). In addition, when used as a therapy post-toxicity, TubA was at least as effective as NAC therapy compared to vehicle-treated animals in reducing AST (p<0.01) and ALT (p<0.05) levels.
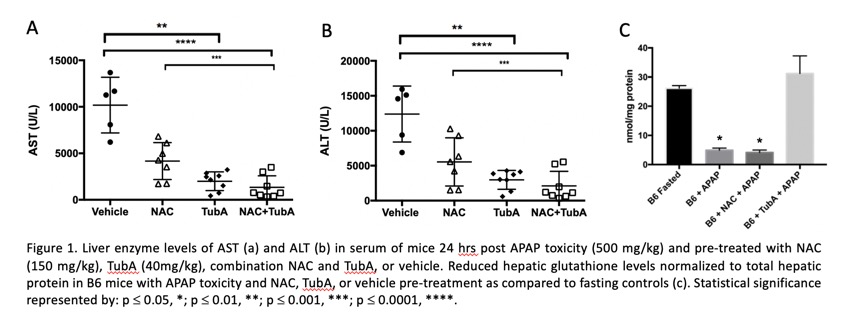
Conclusions: HDAC-6 inhibition mitigates AILI. Treatment with HDAC-6 inhibitor TubA, pre- and post-toxicity had greater than or equal protection as compared to the standard of care NAC treatment, respectively. With further understanding of the underlying mechanisms, HDAC-6 inhibition provides a promising therapeutic mechanism for treatment of AILI and potentially other forms of liver injury thereby reducing the necessity for liver transplantation therapy.
[1] Acute Liver Injury
[2] Hepatotoxicity
[3] Immunosuppression
[4] Acetaminophen Induced Liver Injury