Multicenter prospective cohort study of CMV T cell immunity and first post-transplant CMV DNAemia in pediatric solid organ transplant recipients
Daniel Dulek1, Yilun Sun2, Jose Ferrolino2, Rebecca Pellett Madan3, Jennifer Schuster4, Inci Yildirim5, Tanvi Sharma6, Marc Foca11, Betsy Herold11, William Muller7, Li Tang2, Marian Michaels8, Steven Kleiboeker9, Michael Green8, Lara Danziger-Isakov10.
1Vanderbilt University Medical Center, Nashville, TN, United States; 2St. Jude Children's Research Hospital, Memphis, TN, United States; 3New York University, New York City, NY, United States; 4Children's Mercy Hospital, Kansas City, MO, United States; 5Yale School of Medicine, New Haven, CT, United States; 6Boston Children's Hospital, Boston, MA, United States; 7Lurie Children's Hospital, Chicago, IL, United States; 8University of Pittsburgh Medical Center, Pittsburgh, PA, United States; 9Eurofins-Viracor, Lenexa, KS, United States; 10Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; 11Children's Hospital at Montefiore Einstein, Bronx, NY, United States
Purpose: Cytomegalovirus (CMV) infection morbidity and prophylactic CMV antiviral toxicities remain significant in pediatric solid organ transplantation (PSOT). The utility of CMV T cell immunity (TCI) assays in stratifying post-transplant CMV infection risk has not been well studied in PSOT.
Methods: We conducted a prospective multicenter study of the flow cytometry-based CMV inSIGHTTM T Cell Immunity (TCI) Assay (Eurofins-Viracor). This assay quantifies whole blood CD4+ and CD8+T cell IFNγ expression following CMV antigen mixture or pp65 peptide stimulation. A threshold of >0.20% IFNγ+ cells is defined as a positive response. PSOT recipients (<18 years) who were donorand/or recipient CMV seropositive were enrolled pre-transplant and followed for 12 months post-SOT. We tested the hypothesis that CMV TCI assay positivity measured at 3- and 6- months post-transplant is associated with decreased frequency of subsequent first-episode CMV DNAemia. Statistical analysis was performed using Firth’s penalized logistic regression.
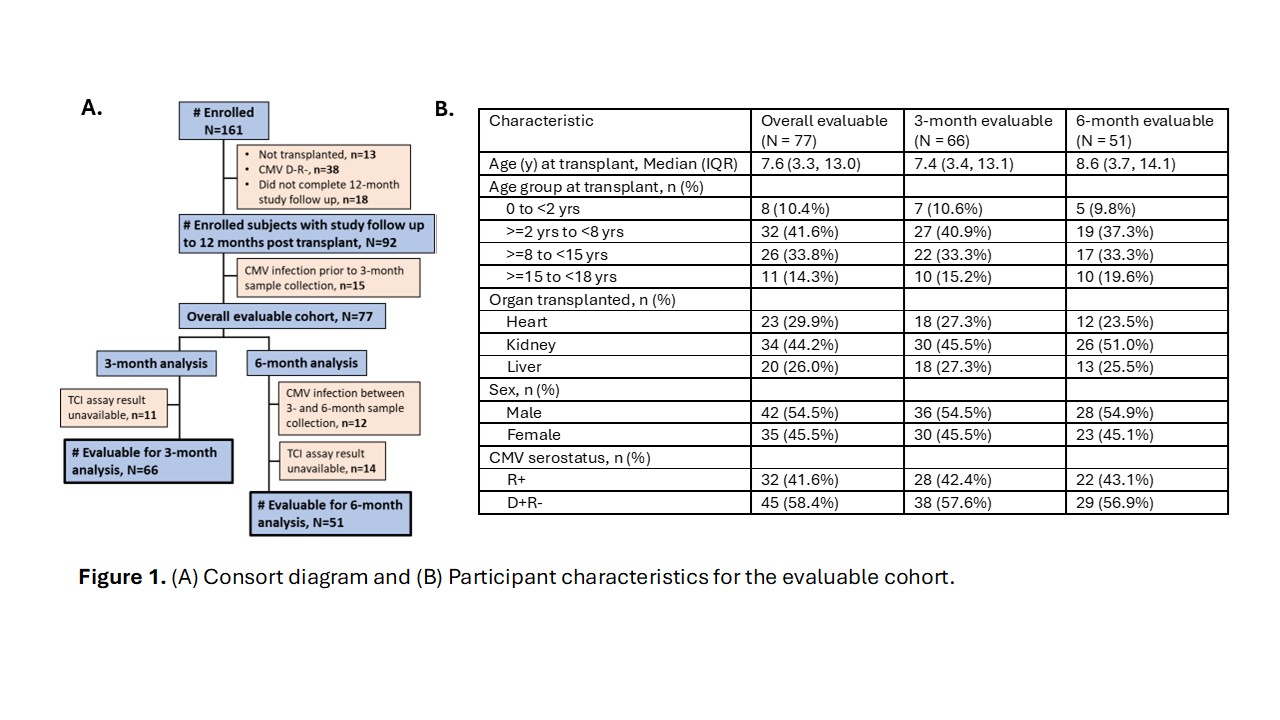
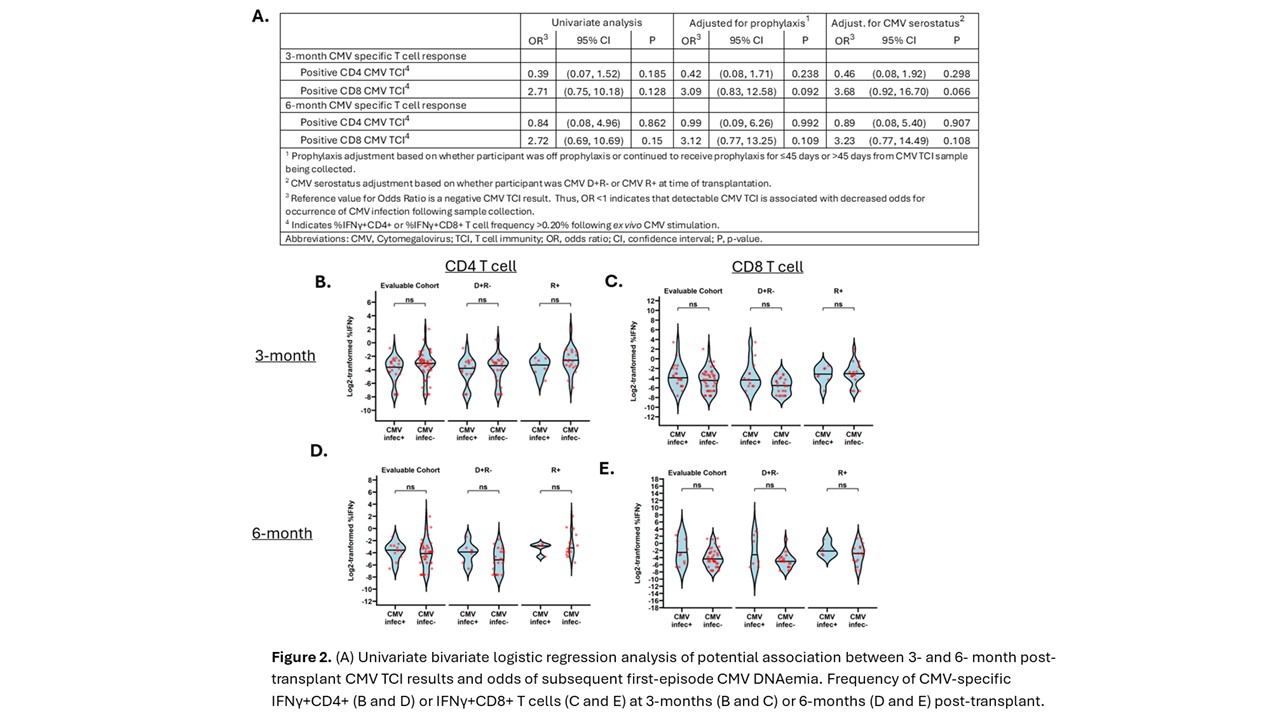
Results: Seventy-seven evaluable participants contributed to this analysis (Figure 1). Twenty-two episodes of first CMV infection were identified (28.6% of participants). Amongst these first-episode infections were 16 asymptomatic CMV DNAemia episodes, five CMV viral syndrome episodes, and one occurrence of CMV disease. Using positivity cutoffs previously defined in adult patients, positive CD4+ or CD8+ CMV TCI at 3 months or 6months post-transplant was not associated with decreased risk of subsequent CMV DNAemia (Figure 2). This lack of association held when adjusting for duration of CMV prophylaxis or for CMVserostatus and when limiting CMV DNAemia event occurrence to 90 days following CMV TCI sample measurement. No significant differences in frequency of IFNγ+ CD4+ or CD8+ T cells were notedwhen compared between participants with or without a first post-transplant episode of CMV DNAemia after the 3- or 6-month CMV TCI timepoints (Figure 2).
Conclusions: CMV TCI measured at 3- and 6-months post-transplantation in PSOT is not associated with decreased risk of subsequent first episode CMV infection within the first-year post-transplantation. Study limitations, including between-site variation in CMV DNAemia screening frequency, may have precluded detecting a significant association. Future analyses will evaluate alternative CMV TCI positivity cutoffs and assess the association between CMV TCI and symptomatic CMV infection (viral syndrome and/or tissue invasive disease)
[1] cytomegalovirus
[2] T cells
[3] pediatric