Prevention of human papillomavirus (HPV) infection in pediatric kidney (KTx) and liver transplant recipients (LTx) and in pediatric patients with advanced chronic kidney disease (CKD): a prospective, multicenter vaccine surveillance trial (HPVaxResponse study)
Britta Höcker1, Alexander Fichtner1, Paul Schnitzler1, Kai Krupka1, Isabella Guzzo1, Lars Pape1, Anja Büscher1, Markus Weitz1, Atif Awan1, Antonia Bouts1, Nikoleta Printza1, Sabine König1, Jun Oh1, Elke Lainka1, Laura Prieto1, Anja Sander1, Peter Sehr2, Michael Pawlita2, Tim Waterboer2, Julia Butt2, Burkhard Tönshoff1.
1CERTAIN Research Network, CERTAIN-LI Research Network, Heidelberg, Germany; 2German Cancer Research Center (DKFZ), European Molecular Biology Laboratory (EMBL), Heidelberg, Germany
Introduction: Due to their immunosuppressive therapy, solid-organ transplant (SOT) recipients bear an increased risk of HPV-associated malignancies compared to healthy individuals. In addition, immunosuppressive medication potentially reduces the immune response to HPV vaccines.
Methods: We therefore performed a prospective, multicenter study to investigate the immune response to HPV vaccination in pediatric KTx, LTx and CKD patients. Type-specific HPV vaccine titers (cLIA) and neutralizing antibodies (PBNA) were measured before as well as 1-3 months and 15-21 months after HPV vaccination.
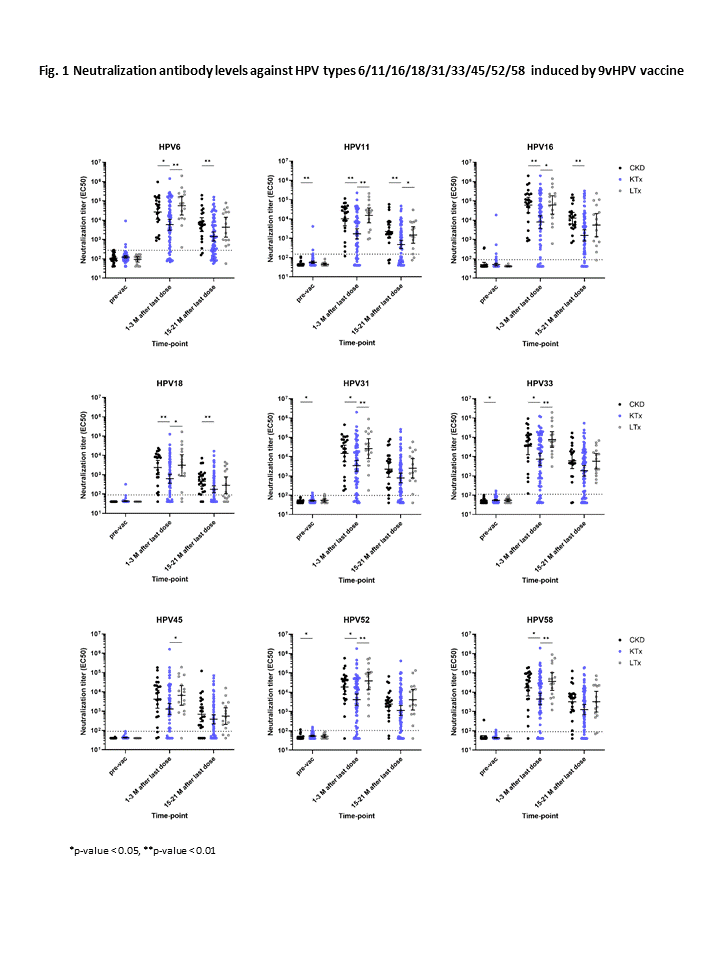
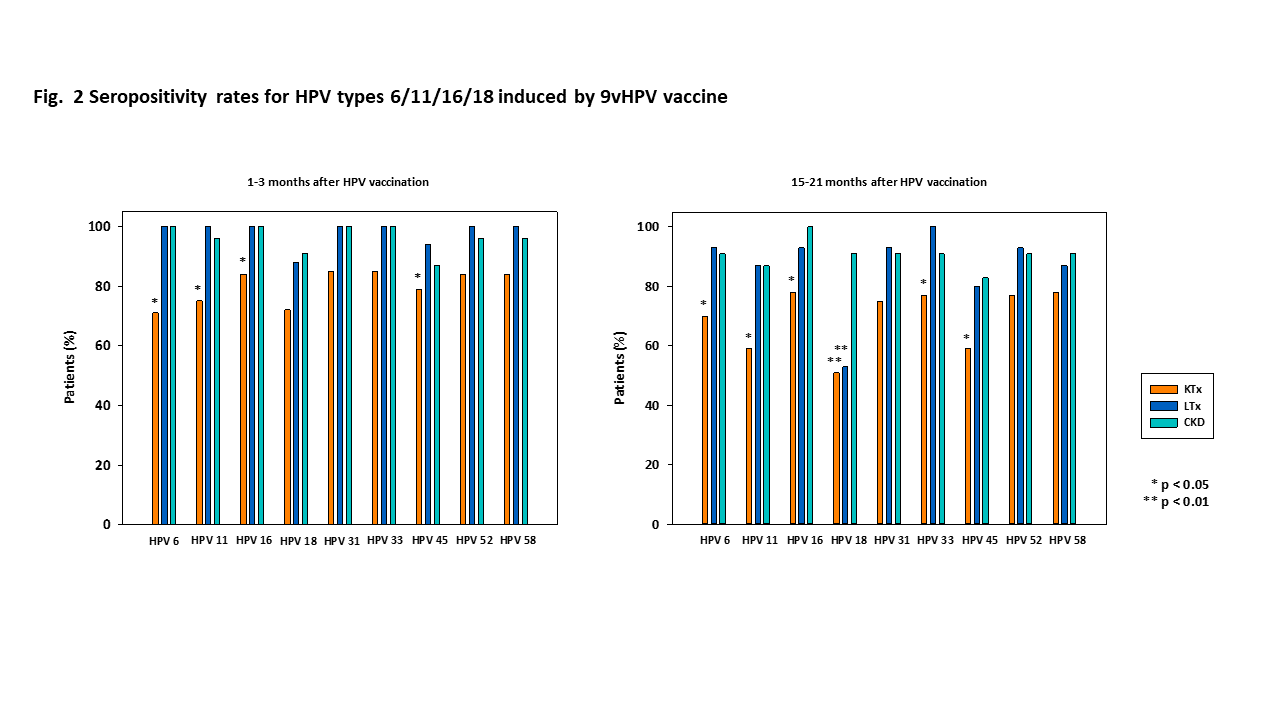
Results: 136 patients (93 KT, 16 LTx, 27 CKD; median age at vaccination 13.4 years), who received a 9vHPV vaccine, were included in this analysis. 66.2% of patients received a 2-dose, and 33.8% a 3-dose vaccination schedule. HPV vaccine titers and neutralizing antibody levels were significantly lower in pediatric KTx than in CKD or LTx patients after vaccination. HPV seropositivity rates were also significantly lower in pediatric KTx than in CKD and LTx patients after vaccination. Depending on the HPV type, 22%-34% of KTx patients did not develop HPV vaccine titers (cLIA) above the cut-off value, defined by the manufacturer. For HPV 18, the immune response to 9vHPV vaccination was lower in both KTx and LTx patients. Female sex (OR 3.8), age at vaccination (OR 1.6), hypogammaglobulinemia (OR 7.3), MMF-based immunosuppression (OR 6.0) and a high overall immunosuppressive score (OR 1.4) were risk factors associated with lower HPV seropositivity rates in pediatric KTx recipients after 9vHPV vaccination.
Conclusions: HPV seropositivity rates and neutralizing antibody titers after 9vHPV vaccination are lower in pediatric KTx than in LTx or CKD patients, due to their more intensive immunosuppressive maintenance therapy. One third of KTx recipients do not develop HPV vaccine titers above the cut-off value. HPV vaccination should be performed early and preferably before transplantation. A 3-dose HPV vaccination schedule should be applied to all transplant recipients, as recommended by national and international guidelines. HPV vaccine titer measurement and additional vaccine doses in non-responders may be considered in KTx patients.
Funding source: The study was financed by MSD Sharp & Dohme GmbH, Germany, with an unrestricted grant. The financier had no role in the study design, study conduct, data analysis, or reporting of results. BH is an awardee of the “DZIF Clinical Leave Stipend” from the German Center for Infection Research (DZIF).
[1] Kidney Transplantation
[2] Liver Transplantation
[3] Chronic Kidney Disease
[4] HPV Vaccination
[5] Immunosuppression
[6] Neutralizing Vaccine Titers
[7] HPV-associated Malignancy