
Safety profile of antiviral prophylaxis for cytomegalovirus in pediatric kidney transplant patients: A systematic review
Ni Nyoman Berlian Aryadevi1, Nathasha Brigitta Selene1, Henny Adriani Puspitasari2, Nina Dwi Putri3.
1Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 2Nephrology Division, Department of Child Health, Faculty of Medicine Universitas Indonesia/Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; 3Infection and Tropical Disease Division, Department of Child Health, Faculty of Medicine Universitas Indonesia/Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia
Introduction: Cytomegalovirus (CMV) is a common opportunistic infection in pediatric kidney transplant recipients and is associated with indirect effects such as impaired graft function, reduced glomerular filtration rate (GFR), and chronic allograft injury. Children are at higher risk due to their frequent seronegative status and the use of lymphocyte-depleting agents for induction therapy. Antiviral prophylaxis, such as valganciclovir, is widely used to prevent CMV infection, yet concerns remain regarding appropriate dosing and safety profile in pediatric patients. This systematic review aims to synthesize current evidence on the adverse effects (AEs) of CMV antiviral prophylaxis in pediatric kidney transplant patients.
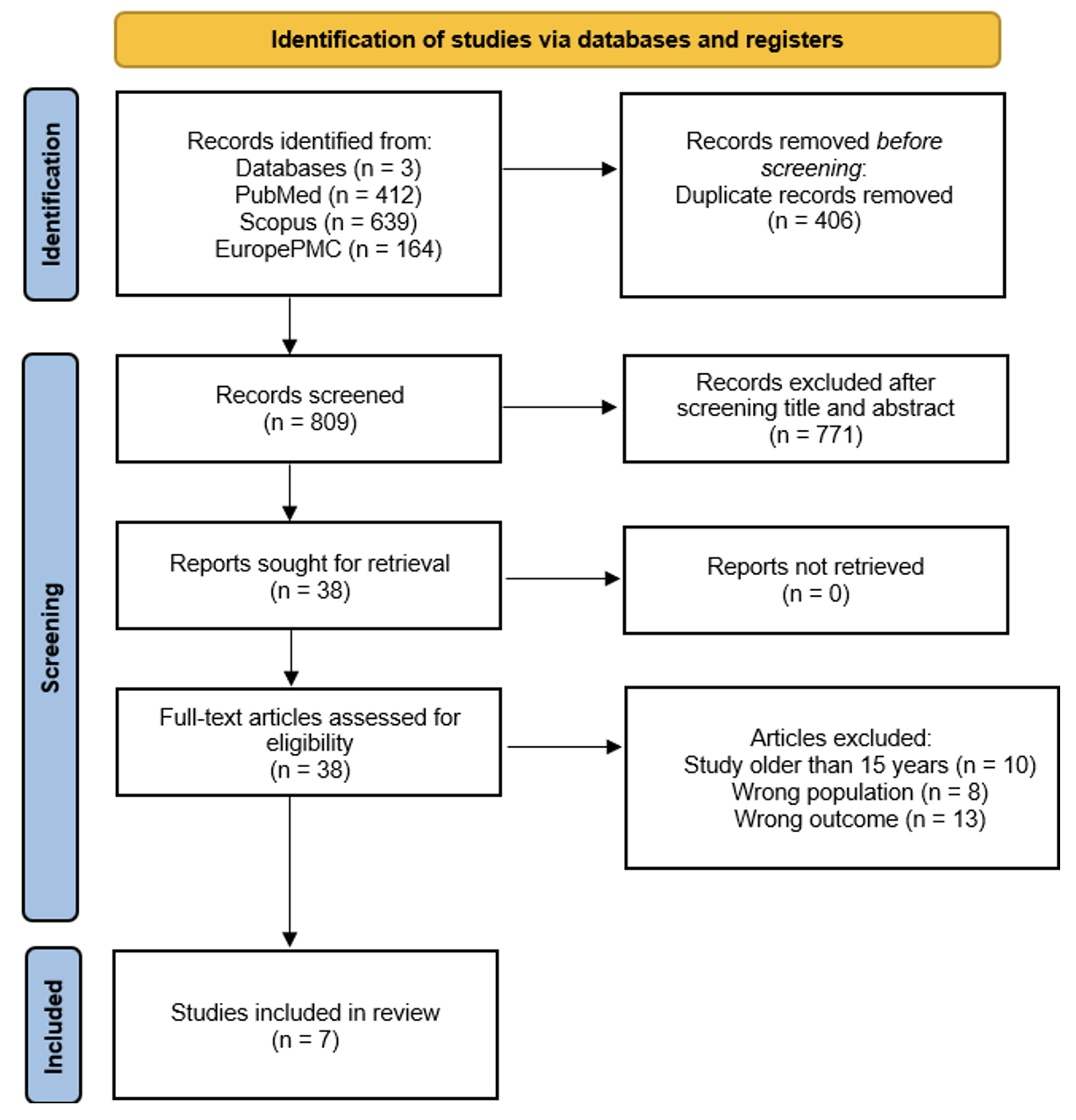
Methods: A comprehensive literature search was conducted in electronic databases (PubMed, Scopus, and EuropePMC) up to March 11, 2025. Eligible studies included clinical research evaluating adverse effects of CMV antiviral prophylaxis in pediatric kidney transplant recipients (<21 years old). Studies published over 15 years ago or in languages other than English were excluded. The quality of included studies was assessed using the Newcastle-Ottawa Scale (NOS).
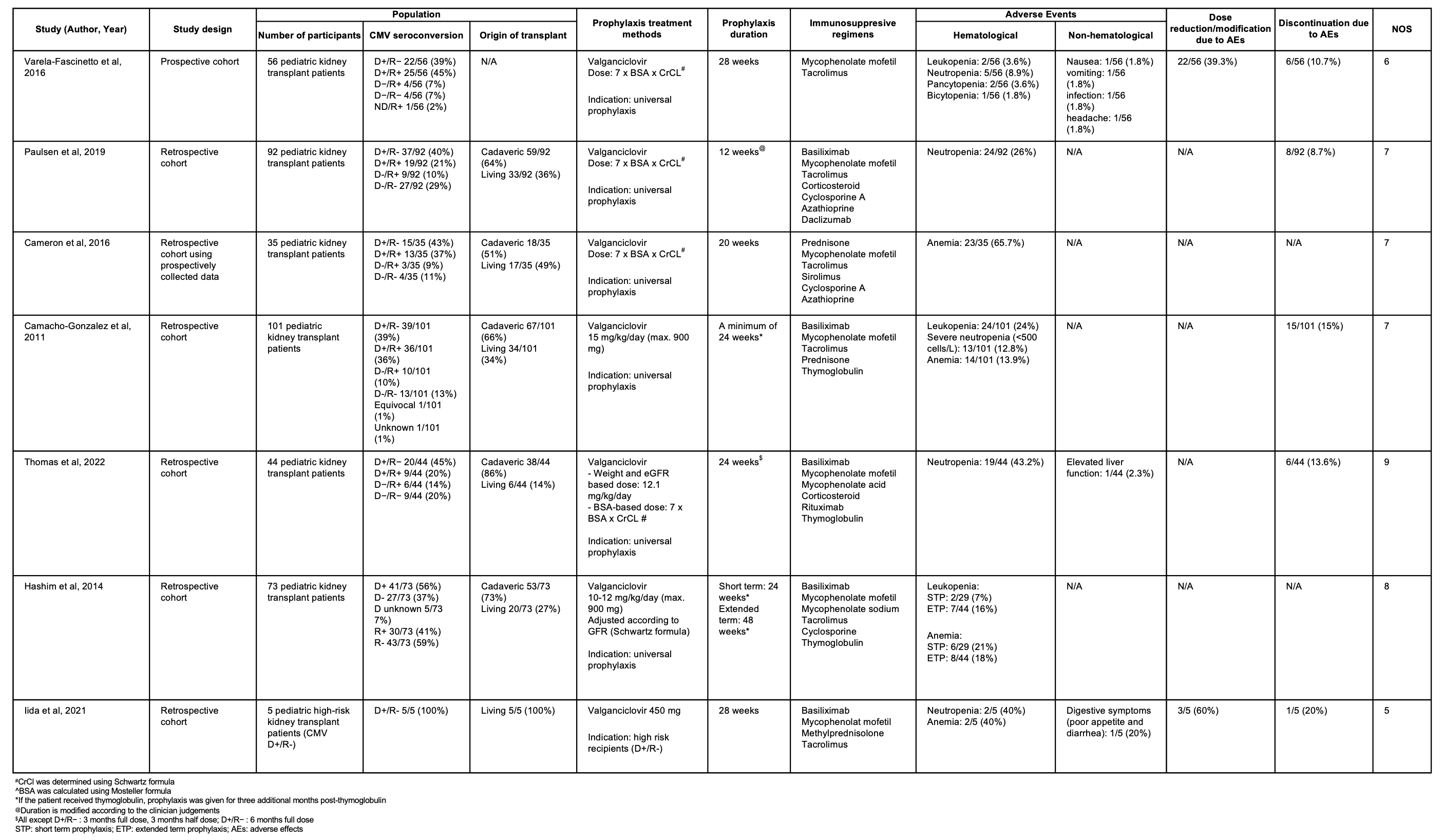
Results: Seven studies involving 406 pediatric kidney transplant recipients (aged 1–20.4 years) were included. Among 350 patients with donor data, 67% received cadaveric and 33% living donor transplants. Six studies used universal CMV prophylaxis, while one provided prophylaxis only to high-risk recipients (D+/R–). Six studies were rated as good quality and one as fair. All used valganciclovir for prophylaxis, with durations ranging from 12 to 48 weeks. Dosing strategies varied: three studies adjusted doses based on body surface area and creatinine clearance; one started with 10–12 mg/kg/day and adjusted to GFR; one used 15 mg/kg/day; one compared weight- and GFR-based dosing to body surface area-based dosing; and one study used a fixed dose of 450 mg for all patients. Hematological adverse effects, particularly neutropenia (8.9%–43.2%) and anemia (13.9%–65.7%) were most common. Other effects included gastrointestinal symptoms, elevated liver enzymes, infections, and headache. Adverse effects led to dose adjustments in 39.3%–60% of patients and discontinuation of prophylaxis in 8.7%–20%.
Conclusion: In pediatric patients receiving universal CMV prophylaxis, close monitoring for adverse effects, particularly hematological toxicity, is essential, as it may require dose adjustments or even discontinuation of the prophylactic treatment. Other potential non-hematological adverse effects include gastrointestinal symptoms, headache, and infections.
[1] Kidney Transplantation
[2] Valganciclovir
[3] Safety
[4] Pediatric
[5] Cytomegalovirus Prophylaxis