
Donor-derived cell-free DNA (dd-cfDNA) as a non-invasive biomarker of kidney allograft rejection in pediatric kidney transplantation
Julien Hogan1,2, Roshan George3, Carissa Hayes3, Olivier Aubert2, Véronique Baudouin1, Elodie Cheyssac1, Charlotte Duneton1, Chris Fan3, Margret Kamel3, Marion Rabant4, Hong Yin5, Alexandre Loupy2, Rouba Garro3.
1Pediatric nephrology, Hopital Robert Debré, APHP, PARIS, France; 2Paris Institute for Transplantation and Organ Regeneration (PITOR), INSERM U970, Paris, France; 3Pediatric nephrology, Children Healthcare of Atlanta, Atlanta, GA, United States; 4Pathology department, Hopital Necker, APHP, PARIS, France; 5Pathology department, Children Healthcare of Atlanta, Atlanta, GA, United States
Introduction: Allograft biopsy remains the gold standard to diagnose rejection. New non-invasive biomarkers are needed to avoid unnecessary biopsies and to diagnose early rejection. We studied the performance of dd-cfDNA to detect rejection in an unselected cohort of pediatric kidney transplant recipients (pKTRs) and determined whether dd-cfDNA could improve standard-of-care monitoring and detection of kidney allograft rejection in children.
Methods: We included 196 pKTRs, who underwent 367 biopsies with concomitant dd-cfDNA assessment. We assessed the association of dd-cfDNA with histological lesions and with rejection using a Cox regression model and compared the discrimination (AUC) of three models: standard-of-care, dd-cfDNA alone, and combined.
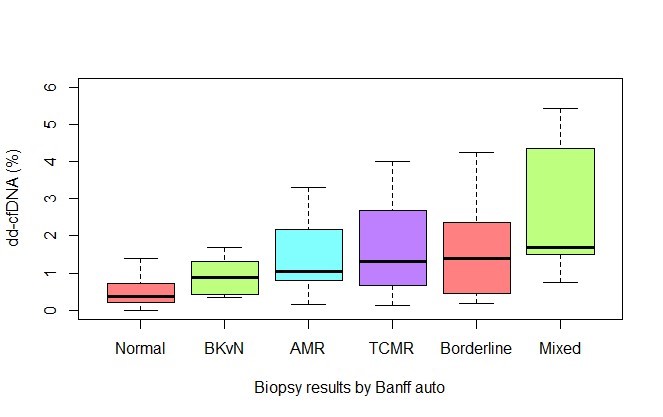
Results: We found a significant increase of dd-cfDNA levels with higher degree of inflammation on the biopsies. dd-cfDNA was strongly and independently associated with the presence of allograft rejection (OR 1.89, 95% CI [1.40;2.60]).
dd-cfDNA alone had a fair discrimination of 0.76, 95% CI [0.69; 0.81] to detect rejection and its addition to standard-of-care resulted in a significant increase in discrimination from 0.80, 95% CI [0.71; 0.85] to 0.84, CI 95% [0.79; 0.90], p=0.01.
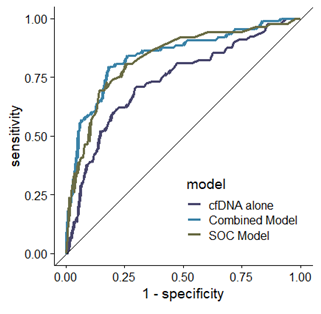
Conclusion: dd-cfDNA combined with standard-of-care improves the prediction of rejection in pKTRs. Further specific studies are needed to better define how to use this promising biomarker in various contexts of use in children.