
Association between donor derived cell free DNA, donor specific antibody, and rejection in pediatric kidney transplant recipients
Lyndsay Harshman1, Arshdeep Kaur1, Kelly Kirshner2, Julia Steinke3, Arundhati Kale4, Erica Winnicki5, Daniel Ranch6, Jayanthi Chandar7, Helen Pizzo2, Paul Hanson8, Ling Shen8, Zunqiu Chen8, Dechu Puliyanda2.
1Stead Family Department of Pediatrics, University of Iowa, Iowa City, IA, United States; 2Cedars Sinai Guerin Children's Hospital, Los Angeles, CA, United States; 3Helen DeVos Children's Hospital, Grand Rapids, MI, United States; 4UC Davis School of Medicine, Davis, CA, United States; 5University of California San Francisco, San Francisco, CA, United States; 6University of Texas Health Science Center, San Antonio, TX, United States; 7University of Miami, Miller School of Medicine, Miami, FL, United States; 8CareDx, San Francisco, CA, United States
Background: The use of donor-derived cell free DNA (dd-cfDNA) in kidney transplantation (KT) is a surrogate biomarker for allograft injury. The value of dd-cfDNA compared to other biomarkers, such as donor-specific antibody (DSA), remains in question, especially among pediatric KT recipients. This multi-center study explored the relationship between dd-cfDNA, DSA, and allograft biopsy data from a cohort of pediatric KT recipients.
Methods: A retrospective data analysis was performed for patients at least 1 year (yr) after KT and without prior history of biopsy-proven rejection. Seven pediatric KT centers in the USA provided data for 265 patients. 184 had renal allograft biopsies with dd-cfDNA (expressed as %) tested within 30 days prior to the biopsy. DSA status (DSA+, DSA-) was based on the most recent DSA result prior to the biopsy and considered unknown if no DSA tested prior to biopsy. Patients with rejections were divided into antibody mediated (AMR), acute cellular rejection (ACR) and mixed rejection. Accounting for repeated measures in the same patients, linear mixed models with dd-cfDNA log2 transformation were used to evaluate the mean difference in log2 (dd-cfDNA) associated with biopsy results and DSA status. The estimated effect is the ratio of mean dd-cfDNA.
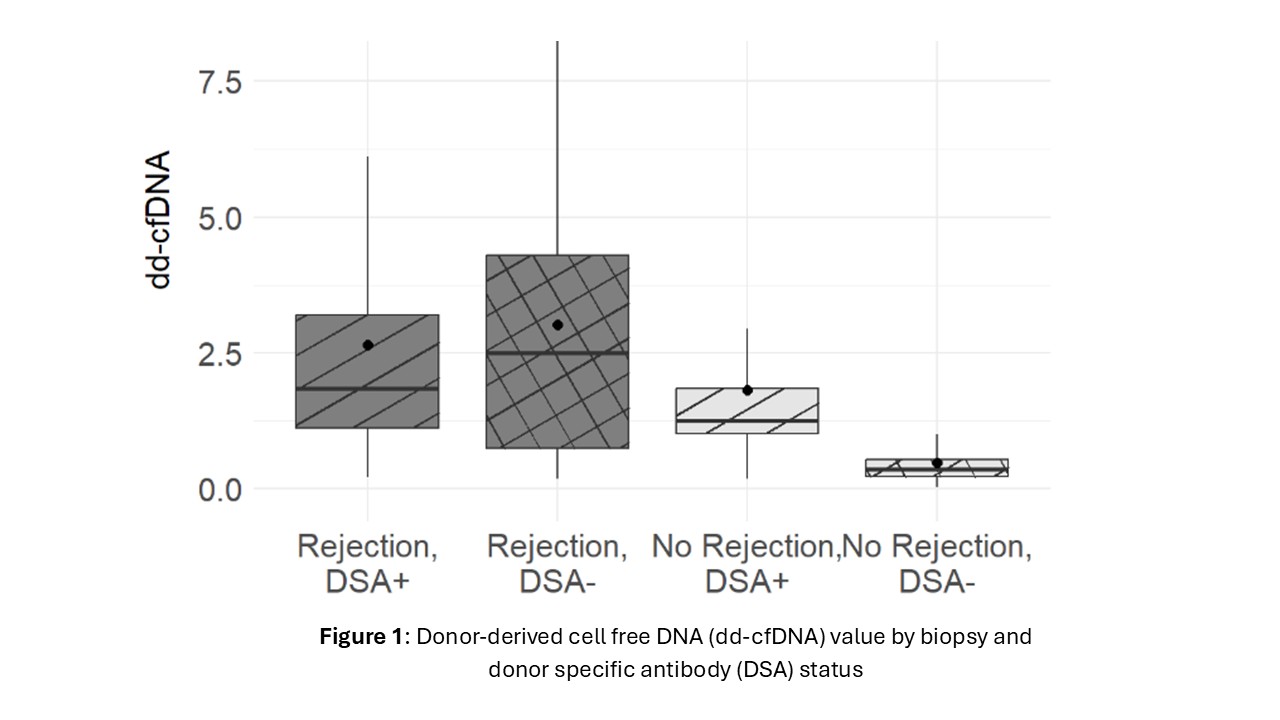
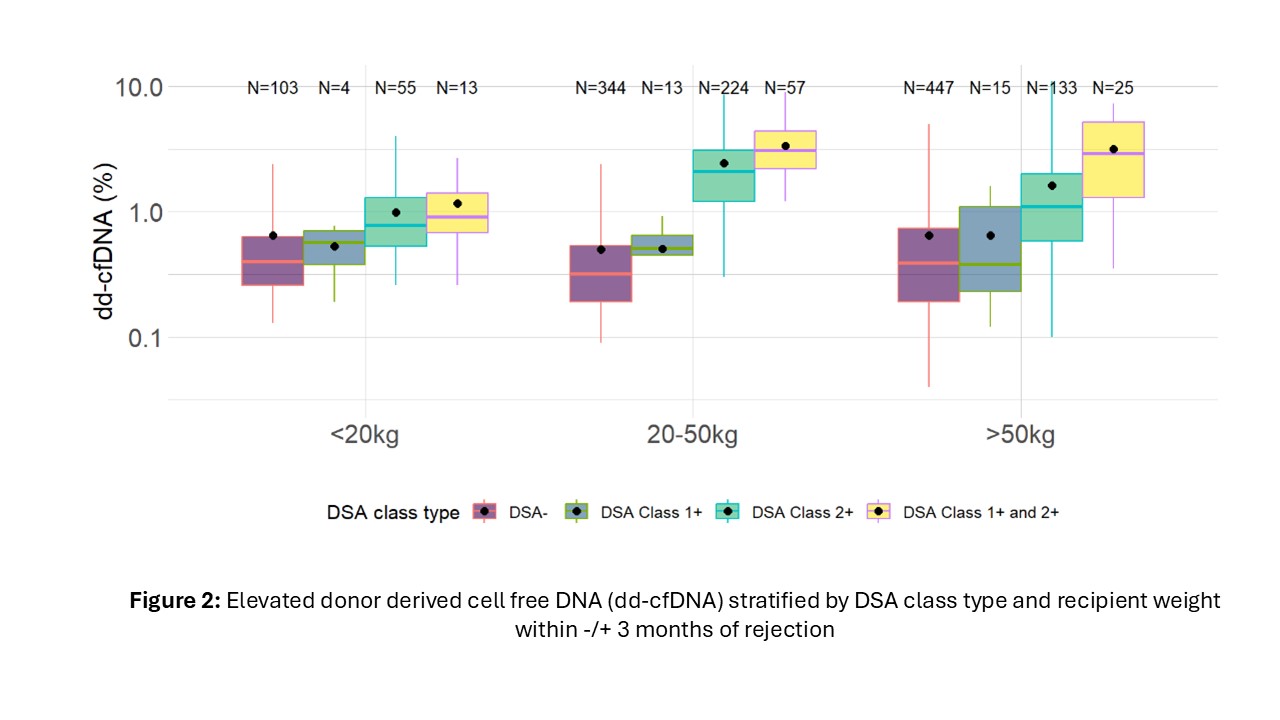
Results: Complete data were available for 151 patients (62.4% male). All recipients were crossmatch negative at the time of transplant. Dd-cfDNA was elevated in patients with rejection regardless of DSA positivity (median1.9% and 2.5% for rejection with and without DSA respectively). In patients with no rejection, DSA positive patients had higher dd-cfDNA compared with DSA negative patients (median 1.3% vs 0.4%) (Figure 1). Among DSA + patients, those with Class II alone and those with both Class I and II DSA had significantly higher dd-cfDNA values than those with only Class 1 such that those with only Class I had similar values to DSA negative patients (Figure 2). Compared to the dd-cfDNA levels at the time of no rejection and DSA-, the mean dd-cfDNA level was 4.2 times higher when rejection with DSA+ (p < 0.001), 4.7 times higher when rejection with DSA- (p < 0.001) and 3.2 times higher when no rejection with DSA+ (p < 0.001). AMR and mixed rejection had higher median dd-cfDNA than ACR with no DSA (2.1% vs 2.0% vs 0.8%); however median dd-cfDNA in patients with ACR and DSA+ (1.7%) were comparable to AMR and mixed rejections. When dd-cfDNA was analyzed among first biopsy status after 6 months from first dd-cfDNA result, dd-cfDNA was elevated up to 4 months prior to AMR compared with ACR or no rejection (median 2.4% vs 0.36% and 0.4%).
Conclusions: DSA positivity is associated with elevated dd-cfDNA with or without rejection. Monitoring serial dd-cfDNA may have a higher utility in the detection of rejection compared to DSA monitoring alone among pediatric KT recipients.
[1] cell free DNA
[2] rejection
[3] pediatric
[4] kidney