Pegcetacoplan for posttransplant patients with complement 3 glomerulopathy or primary (Idiopathic) immune-complex membranoproliferative glomerulonephritis
Andrew S Bomback1, David Kavanagh2, Daniel Zecher3, Julien Zuber4, Ondrej Vicklicky5, Li Li6, Luis Lopez-Lazaro7, Fadi Fakhouri8.
1Irving Medical Center, Columbia University , New York, NY, United States; 2National Renal Complement Therapeutics Centre, Newcastle University, Newcastle, United Kingdom; 3University Hospital Regensburg, University of Regensburg, Regensburg, Germany; 4Department of Kidney and Metabolic Diseases, Transplantation and Clinical Immunology, Necker Hospital, Assistance Publique Hôpitaux de Paris (AP-HP), Paris, France; 5Department of Nephrology, Institute for Clinical and Experimental Medicine, Prague, Czech Republic; 6Apellis, Apellis Pharmaceuticals Inc, Waltham, MA, United States; 7Sobi, Swedish Orphan Biovitrum, Basel, Switzerland; 8Centre Hospitalier Universitaire Vaudois, Lausanne University Hospital, Lausanne, Switzerland
Aim: Complement 3 glomerulopathy (C3G) and primary (idiopathic) immune-complex membranoproliferative glomerulonephritis (IC-MPGN) are rare, chronic kidney diseases that often recur after transplantation despite conventional immunosuppression. NOBLE (Phase 2, NCT04572854) and VALIANT (Phase 3, NCT05067127) showed efficacy and favorable tolerability of pegcetacoplan (C3/C3b inhibitor) for adults and adolescents with native or posttransplant recurrent C3G or primary (idiopathic) IC-MPGN. Here, we describe pegcetacoplan for kidney transplant recipients in these studies.
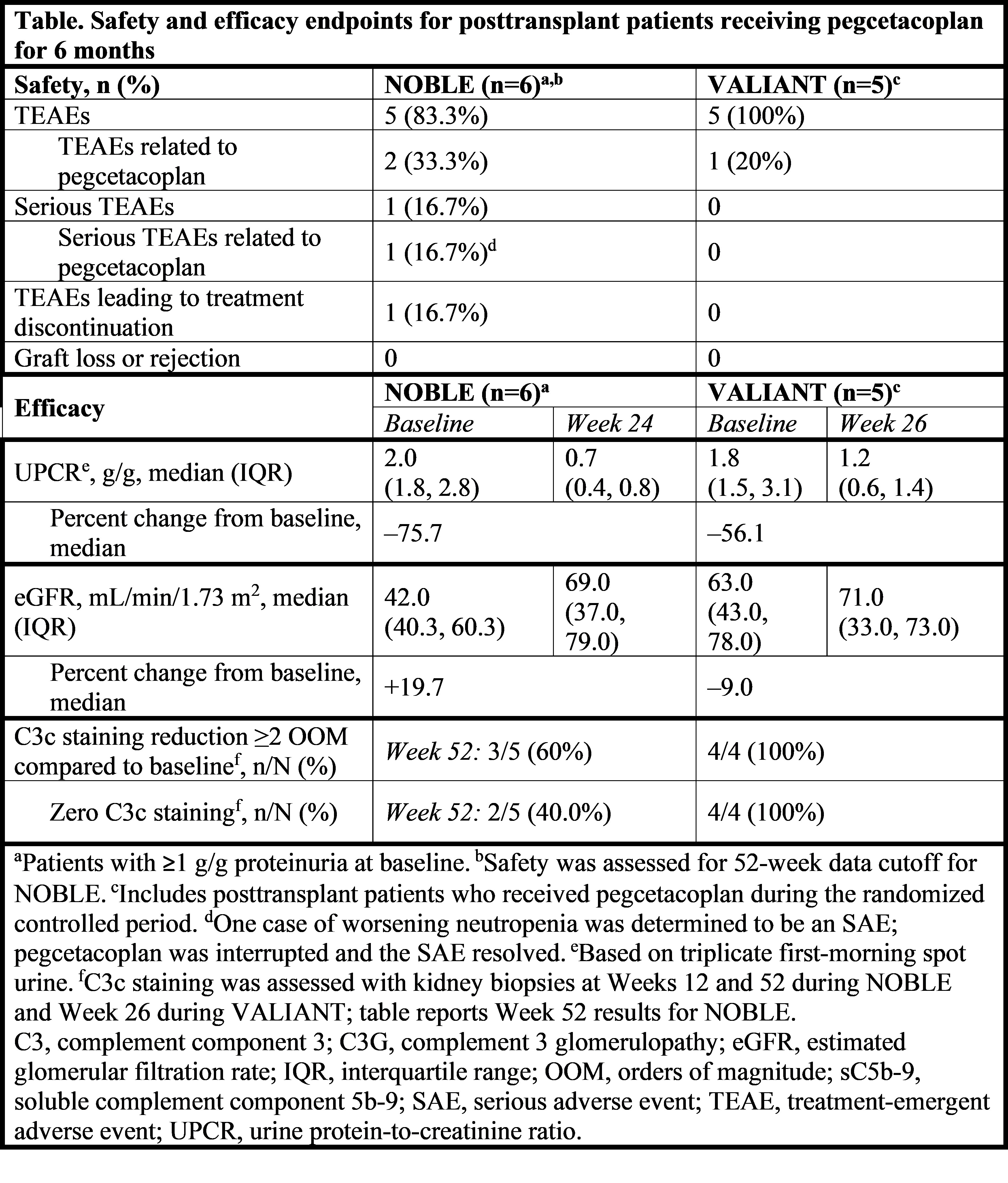
Methods: Eleven posttransplant patients with ≥1 g/g proteinuria at baseline received pegcetacoplan (NOBLE, n=6; VALIANT, n=5). Study designs and biopsy schedules differed between studies, but all patients received pegcetacoplan 1080 mg subcutaneously twice weekly for at least 24–26 weeks. Efficacy endpoints included change from baseline in proteinuria, estimated glomerular filtration rate (eGFR), and C3c staining on kidney biopsy. Treatment-emergent adverse events were also reported.
Results: The safety profile of pegcetacoplan was favorable for posttransplant patients, with no graft loss or rejection reported during 6 months of treatment. Pegcetacoplan-treated patients demonstrated decreased proteinuria and stable eGFR in both studies (Table). C3c staining reduction was observed at Weeks 12 and 52 in NOBLE, and at Week 26 in VALIANT, suggesting that pegcetacoplan leads to early and sustained histopathological improvements.
Conclusions: Six months of pegcetacoplan treatment was safe and well tolerated in posttransplant patients with C3G and primary IC-MPGN. Patients achieved decreased proteinuria, stable eGFR, and decreased C3 staining.
The authors acknowledge the patients, NOBLE and VALIANT investigators, and all other collaborators.
[1] Proteinuria
[2] eGFR
[3] Glomerulonephritis