GFR stabilizes and donor specific antibody levels fall after adding belatacept to a low dose tacrolimus/ mycophenolate/ prednisone regimen in nonadherent pediatric and adolescent kidney transplant recipients
Niloufar Nikfarjam1, Abanti Chaudhuri1, Paul Grimm1, Ruby Patel1.
1Pediatric Nephrology, Stanford University, Palo Alto, CA, United States
This review is to understand benefit in patients transitioned from oral immunosuppressive agents to monthly IV belatacept infusion with tapering dose calcineurin inhibitor (CNI) therapy.
This is a retrospective chart review of 28 patients initiated on belatacept because of nonadherence, poor supervision and/or rejection. Patients were confirmed to be EBV IgG positive and QuantiFERON checked for latent TB prior to initiation of belatacept. Belatacept IV was dosed at 5mg/kg every 4 weeks. While on belatacept tacrolimus targets were 7-10mg/dL if patient had cellular rejection (CR) otherwise 5-7mg/dL for months 0 to 6, then 3-5mg/dL for months 6 to 9 and then ~ 2mg/dL for 9 months and after. Patients remained on other immunosuppression of steroids, mycophenolate and/or azathioprine.
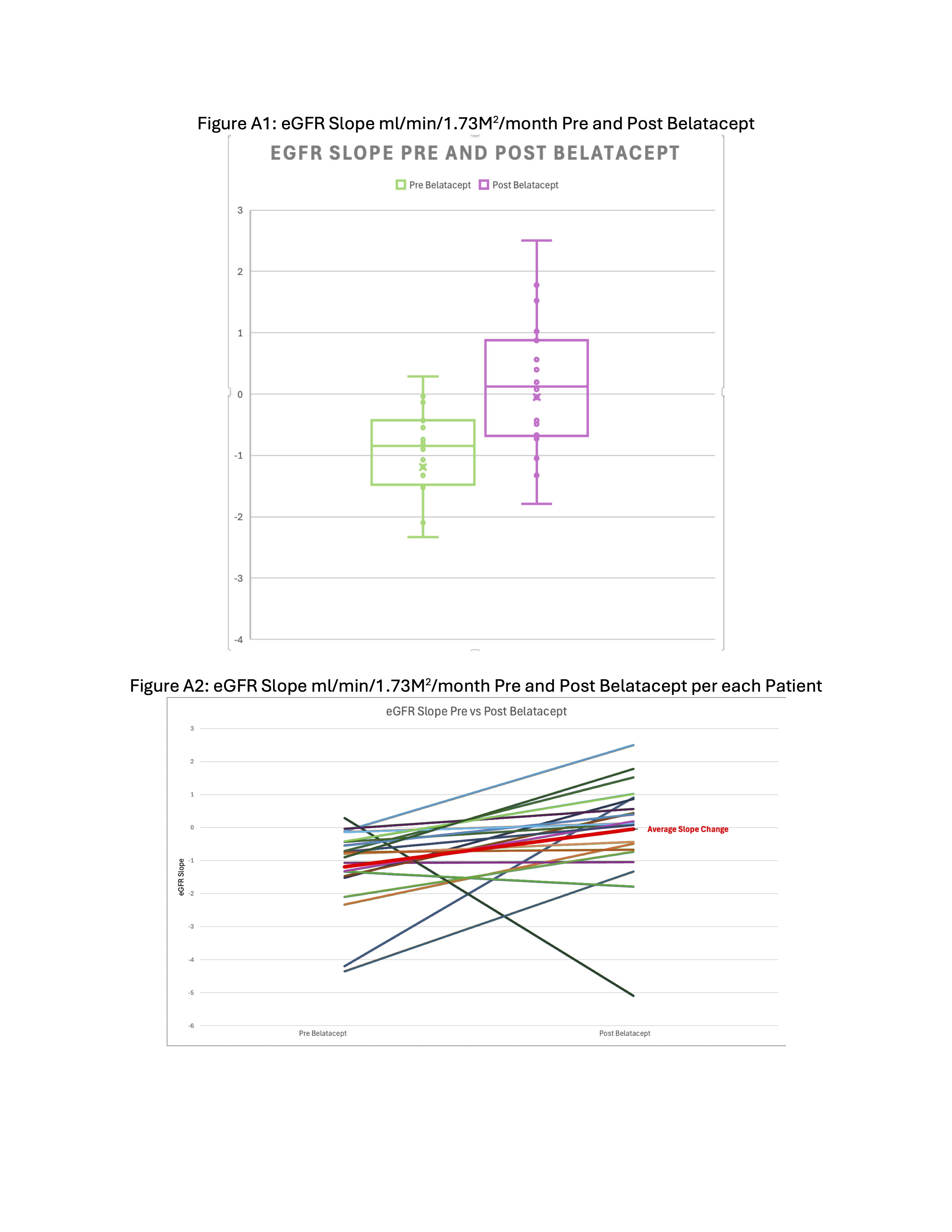
At the time of analysis 28 patients had started belatacept at an average of 15.6 years of age (7-22yrs) and 4.25 years (2-19yrs) post kidney transplant. A total of 21 patients had eGFR data 24months pre and a minimum of 3months to 24months post belatacept. The mean eGFR slope pre was -1.19 ml/min/1.73M2/month (SD 1.22) and post improved to -0.051 ml/min/1.73M2/month (SD 1.56) (95% CI of the differences in the mean -2.04 to -0.24).
The tacrolimus coefficient of variation remained >30% in 19 of 21 patients pre and in 18 of 21 patients post-belatacept.
Follow-up kidney biopsies were performed at a mean of 8months (0 to 23months) after initiation of belatacept in 12 patients. 3 had no change, 6 had improvement and 3 had worsening in frequency/severity of CR. And 8 of 12 had no change, 2 of 12 had improvement, and 2 of 12 had worsening antibody mediated rejection (AMR).
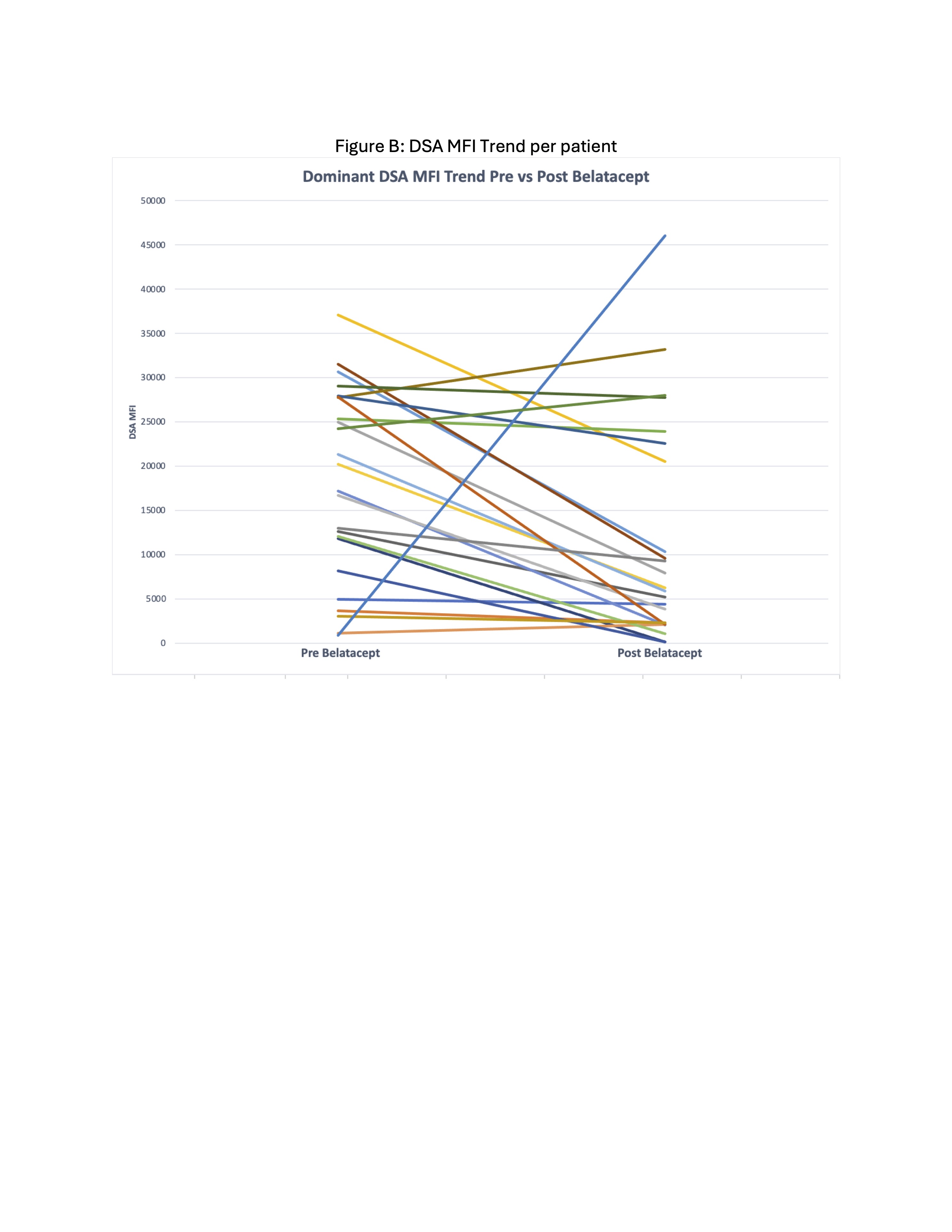
Of the 23 patients with donor specific antibody (DSA) data, 21 had a decrease in the MFI of the immunodominant DSA following belatacept initiation by an average of 51%. 1 patient had DSAs that remained negative pre and post and 1 patient developed a new DSA 6months post.
BK viral blood PCR showed, 16 of 21 patients remained negative, 2 had stable, 2 improved and 1 worse BK levels following initiation of belatacept. CMV blood PCR showed 10 of 21 patients remained negative, 5 had stable, 5 improved and 1 worse. For EBV, 8 of 19 patients remained negative, 5 had stable, 5 improved and 1 worse.
Following initiation of belatacept eGFR slopes were better than pre. This improvement was seen even with majority of patients still having high tacrolimus coefficient of variation. In our cohort almost all patients did not experience worsening viremia post belatacept. And after transitioning to belatacept, the biopsy in 12 patients did not show worsening AR or CR. Most experienced substantial improvement in MFI values of immunodominant DSAs after transitioning to belatacept. Therefore, belatacept along with tapering to low dose CNI may be an effective immunosuppression strategy to stabilize kidney function, reduce DSA and possibly prolong the graft survival in this high risk population.
[1] Immunosuppression
[2] Kidney transplant
[3] Pediatric
[4] GFR
[5] Allograft survival