De novo use of tacrolimus granules in pediatric kidney transplant recipients: Integrated safety and efficacy results from two multicenter open-label studies in China
Longshan Liu1, Zhigang Wang2, Lan Zhu3, Yonghua Feng2, Xubiao Xie4, Gang Chen3, Jun Zhang5, Yue Li5, Changxi Wang1.
1Organ Transplant Center, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, People's Republic of China; 2The Department of Kidney Transplantation, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People's Republic of China; 3Institute of Organ Transplantation, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People's Republic of China; 4Department of Kidney Transplantation, Second Xiangya Hospital, Central South University, Changsha, People's Republic of China; 5Astellas (China) Investment Co., Ltd., Beijing, People's Republic of China
Introduction: Data on the use of tacrolimus granules for immunosuppression in Asian pediatric patients are limited. We conducted two open-label, multicenter, single-arm, phase IV studies in China to assess safety and efficacy of tacrolimus granules (Modigraf®, MOD) in pediatric patients after kidney transplant (KTx).
Methods: Patients aged <18 years who underwent de novo allograft KTx in participating centers in China were prospectively enrolled between December 2021 and March 2023 to receive MOD for 12 months. Initial tacrolimus dose was 0.2 mg/kg/day or 0.15–0.3 mg/kg/day orally; subsequent doses were adjusted by the investigator based on clinical evidence of efficacy, occurrence of adverse events, and tacrolimus whole blood trough level (recommended range: 5–15 ng/mL). Both studies evaluated MOD based on incidence of acute rejection (biopsy-proven or clinically suspected), graft and patient survival rates, dose adjustments, tacrolimus trough levels, and incidence of treatment-emergent adverse events (TEAEs).
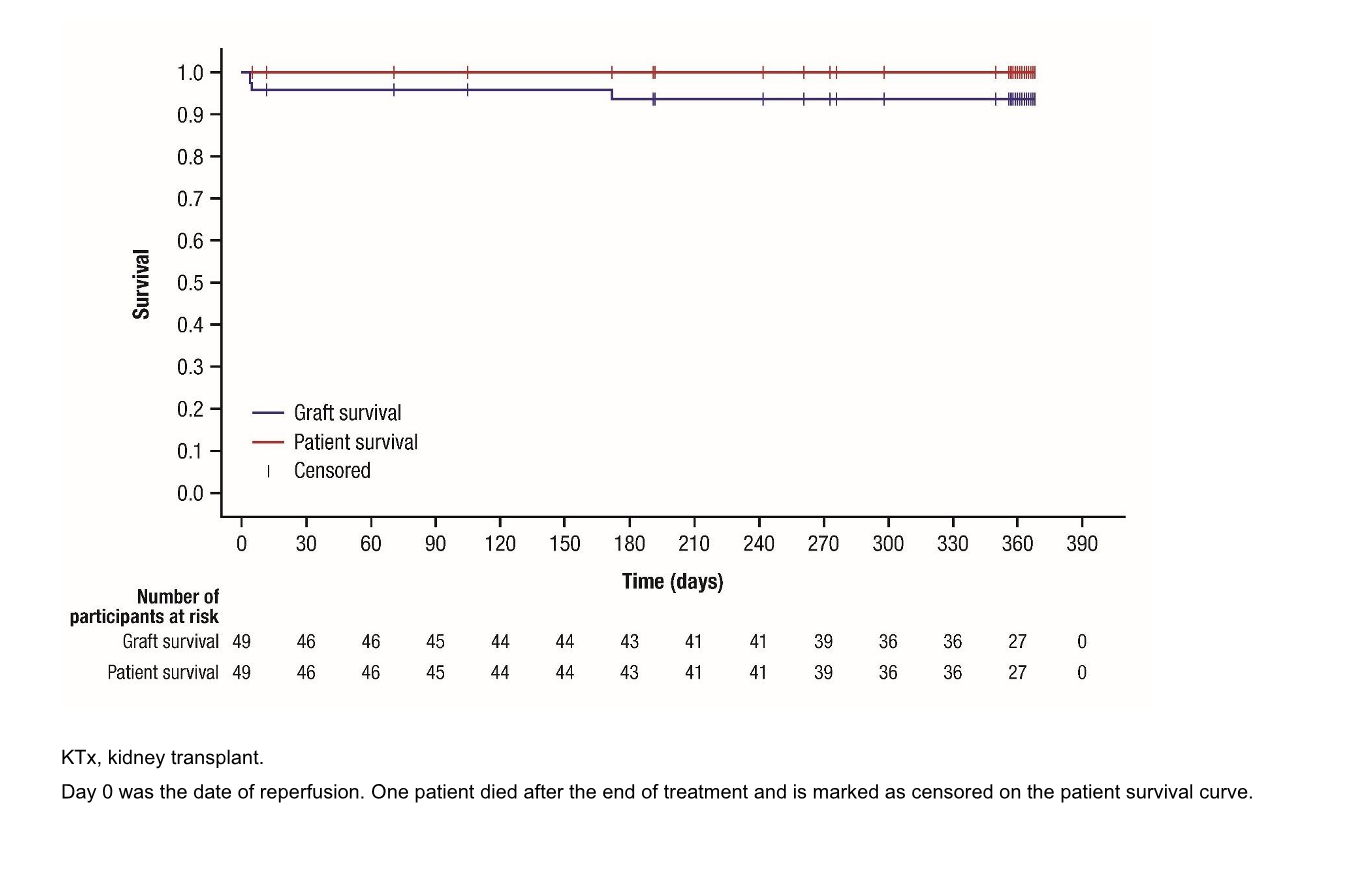
Results: Across both studies, 50 patients were enrolled and 49 received treatment. Most (71.4%, 35/49) patients were male and the median (range) age was 13 (2–17) years. Almost all (95.9%, 47/49) received an organ from deceased donor. Mean (SD) tacrolimus dose was 0.170 (0.111) mg/kg/day; mean (SD) number of dose adjustments was 13.5 (5.3). Mean (SD) tacrolimus whole blood trough level was 8.19 (1.33) ng/mL. Incidence of acute rejection was 4.1% (2/49 patients; 95% CI: 0.5–14.0%), while graft and patient survival rates were 93.9% (46/49) and 98.0% (48/49), respectively (Figure 1). Incidence of drug-related TEAEs was 73.5% (36/49); 10.2% (5/49) patients reported drug-related TEAEs leading to treatment withdrawal. No new safety issues were identified.
Conclusion: MOD had an acceptable tolerability profile when used within recommended trough levels in pediatric KTx recipients for ≤12 months. Incidence of acute rejection and graft loss was numerically lower than historical data in this population.
This study was funded by Astellas Pharma Inc. Editorial support was provided by Iona Easthope, DPhil, of Lumanity, funded by Astellas Pharma Inc..