Sepsis and disseminated primary varicella in a vaccinated multivisceral transplant recipient
Julie Iglesias1, Katherine Sweeny2, Christine Lee2, Lakshmi Ganapathi3.
1Pediatric Transplant Center, Boston Children’s Hospital, Boston, MA, United States; 2 Division of Gastroenterology, Hepatology and Nutrition, Boston Children’s Hospital/Harvard Medical School, Boston, MA, United States; 3Division of Pediatric Infectious Diseases and Global Health, Massachusetts General Hospital/Harvard Medical School , Boston, MA, United States
Introduction: This is a case report of a vaccinated multivisceral transplant recipient 12 years post-transplant who developed disseminated varicella zoster virus (VZV) infection with likely secondary bacterial sepsis. This occurred despite him receiving two doses of varicella (VZV) vaccine as a child prior to transplantation.
Case Description: The patient is a 25-year-old male with a history of hepatocellular carcinoma who underwent a multivisceral transplant (stomach, duodenum, pancreas, liver, and small intestine).
His post-transplant course was complicated by multiple episodes of rejection requiring intensified immunosuppression.
Four days prior to presentation, he reported having significant back pain. He was seen at a local emergency department and had a CT that showed a prolapsed intervertebral disc He was discharged to home with a muscle relaxant, with no improvement in his pain. Two days later, he developed vesicular rash, appearing in crops, spreading cephalocaudally and involving oral mucosa. He re-presented to local emergency department with additional symptoms of confusion, fever and diarrhea.
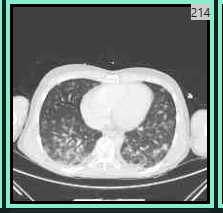
He was transferred urgently to our tertiary care hospital where he required intubation and vasopressors. A comprehensive infectious disease and dermatologic evaluation yielded a positive vesicle VZV PCR (polymerase chain reaction ) and VZV viremia The quantitative blood PCR was 3,500,000 copies/ml. The patient’s hospital course was complicated by varicella pneumonia, and elevated transaminases. A CT chest was notable for randomly distributed bilateral coalescing nodular opacities, with a surrounding halo of ground glass opacity as can be seen with VZV pneumonitis. For disseminated VZV, he was given intravenous acyclovir for a six week course and then transitioned to Valacyclovir suppression. Given initial concern for superimposed bacterial sepsis he also received a 2-week course of broad-spectrum antibiotics.
Conclusion: Although a rare complication after multivisceral transplant, varicella should be considered on the differential diagnosis of rash in an immunocompromised patient, despite immunization status. Prompt initiation of antiviral therapy remains crucial in decreasing morbidity and mortality from this potentially fatal disease. Given this patient’s significant immunosuppression, he is a candidate for receipt of recombinant zoster vaccine to prevent future herpes zoster. New vaccine approaches for VZV are needed for multivisceral transplant recipients.